We use cookies to ensure that we give you the best experience on our website. By using this website you agree to the Privacy Policy and Terms of Use.
Pipeline
Science
1. We plan to commence clinical trials in fibrosis and/or oncology pending IND submission and FDA feedback.
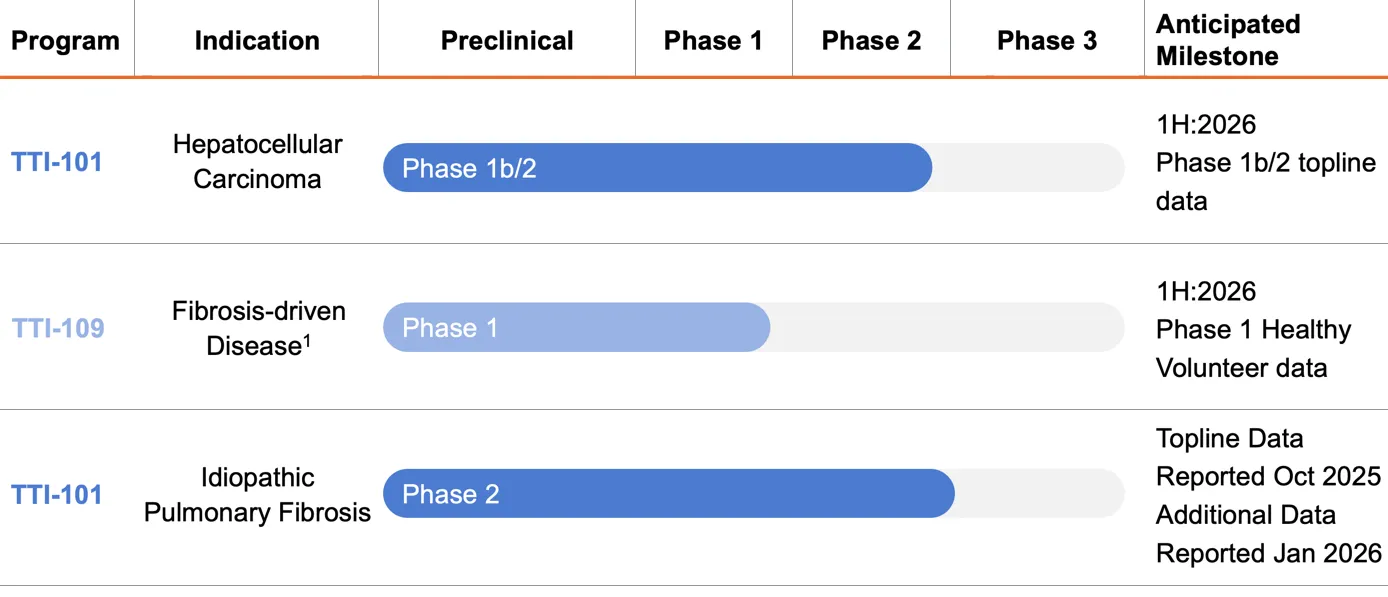
Tvardi’s oral small molecule inhibitors of STAT3 specifically block pY-STAT3. They bind tightly to the SH2 domain of STAT3, which specifically blocks its ability to bind to signaling complexes that contain tyrosine kinases. This is designed to prevent STAT3 from being phosphorylated at tyrosine (Y) 705 and further prevent STAT3 dimerization and nuclear translocation. The selective binding of TTI-101 to the SH2 domain thus inhibits STAT3’s canonical nuclear function, while preserving its essential non-canonical functions associated with cellular respiration within the mitochondria. The FDA has granted orphan drug designation for TTI-101 in both IPF and HCC as well as Fast-Track Designation for TTI-101 in HCC.
Tvardi is currently conducting a Phase 2 clinical trial to investigate TTI-101 as monotherapy and in combination with standard of care (SoC) in patients with hepatocellular carcinoma (HCC). This study is an open-label clinical trial, designed to investigate the safety and efficacy of TTI-101 across three cohorts of patients with HCC: as monotherapy and in combination with SoC treatments pembrolizumab or atezolizumab + bevacizumab.
TTI-109, is also an oral, small molecule STAT3 inhibitor that is structurally related to, yet chemically distinct from, TTI-101 and is designed to enhance the Company’s ability to target STAT3. Tvardi is currently conducting a Phase 1 trial of TTI-109 in healthy volunteers to evaluate safety, tolerability, and pharmacokinetics, as well as bioequivalence to TTI-101.